Loading...
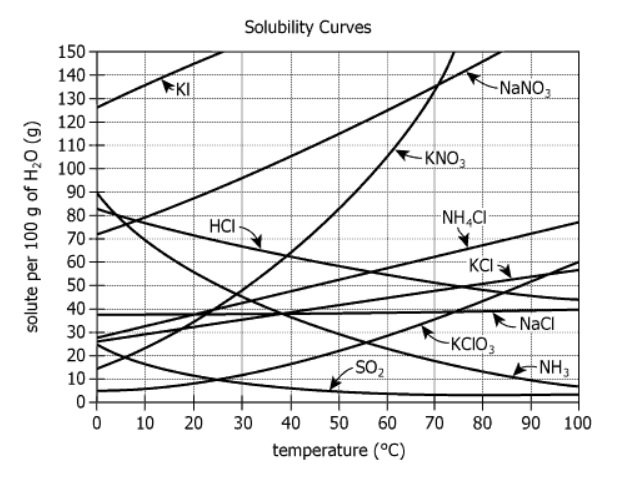
The graph shows how the solubility of various chemicals varies with temperature. The solubility is measured as grams of dissolved chemical (solute) per 100 grams of water.
Under the assumption that the curves shown continue in the same general shape beyond the plotted area, use the drop-down menus to complete each of the following statements in the manner that most accurately reflects the information provided.
| Text Component | Literal Content | Simple Interpretation |
|---|---|---|
| Subject Matter | The graph shows how the solubility of various chemicals varies with temperature. | The chart presents how much of each chemical dissolves in water as temperature changes. |
| Measurement Unit | The solubility is measured as grams of dissolved chemical (solute) per 100 grams of water. | The quantity dissolved is reported per \(100\mathrm{g}\) of water, for each chemical at each temperature. |
| Extrapolation Instruction | Under the assumption that the curves shown continue in the same general shape beyond the plotted area... | When answering, imagine the curves continue in the same way beyond the displayed region. |
| Chart Component | Description | Implication |
|---|---|---|
| Chart Type | Multi-line graph with curves representing chemicals' solubility. | Allows comparison of solubility trends for various chemicals over the temperature range. |
| X-axis | Temperature (\(0°\mathrm{C}\) to \(100°\mathrm{C}\), \(10°\mathrm{C}\) intervals). | Shows how solubility changes as temperature increases from freezing to boiling water. |
| Y-axis | Solubility (grams per \(100\mathrm{g}\,\mathrm{H_2O}\), \(0\text{-}150\mathrm{g}\)). | Can observe both highly and poorly soluble compounds in the same frame. |
| Curve Labels | Each curve labeled directly (KI, NaNO3, KNO3, HCl, NH4Cl, KCl, NaCl, KClO3, SO2, NH3). | Data for each chemical can be read immediately without legend confusion. |
| Pattern/Trends | Some curves strongly upward, some nearly flat, two slope downward. | Indicates drastic differences in how temperature affects solubility of each chemical. |
"At \(60°\mathrm{C}\), the most soluble of the chemicals is most likely _______."
What we need to find: Which chemical's curve reaches the highest point on the y-axis when \(\mathrm{x} = 60°\mathrm{C}\)
To find the most soluble chemical at \(60°\mathrm{C}\), I'll examine where a vertical line at \(\mathrm{x} = 60°\mathrm{C}\) intersects each curve from the answer choices:
Reading the y-values at \(60°\mathrm{C}\):
Comparing these values, KI clearly has the highest solubility at \(60°\mathrm{C}\), positioned significantly above all other chemicals in the answer choices.
"There is most likely a temperature above \(100°\mathrm{C}\) at which NH4Cl is exactly as soluble as _______."
Answer choices available
What we need to find: Which chemical's curve, when extended beyond \(100°\mathrm{C}\), will intersect with NH4Cl's curve
At \(100°\mathrm{C}\), NH4Cl reaches approximately \(75\mathrm{g}\) per \(100\mathrm{g}\) water and continues rising steadily.
Examining the three answer choices at \(100°\mathrm{C}\) and their trends:
To intersect with NH4Cl above \(100°\mathrm{C}\), a curve must be:
KClO3 shows the steepest upward trend of the three choices. Its accelerating rate of increase indicates it will overtake and intersect NH4Cl's more moderate growth rate when extended beyond \(100°\mathrm{C}\).
By systematically reading the solubility values at \(60°\mathrm{C}\), we identified KI as the most soluble chemical at that temperature. Through visual extrapolation of curve trends, we determined that KClO3's steep upward trajectory will lead it to intersect with NH4Cl's solubility curve at some temperature above \(100°\mathrm{C}\). The key insight is using direct value comparison for data within the chart range and trend analysis for predictions beyond it.
These blanks are independent. Blank 1 asks about identifying the most soluble chemical at a specific temperature (\(60°\mathrm{C}\)), while Blank 2 asks about predicting curve intersections beyond the displayed range. Each blank requires a different analytical approach and examines different aspects of the solubility curves without the answers being related to each other.