Loading...
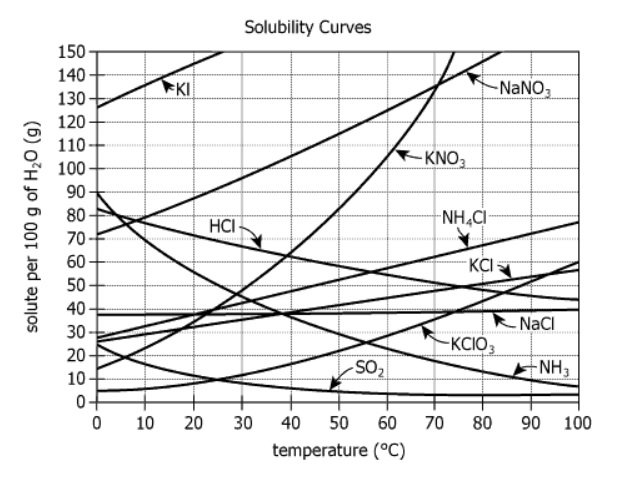
The graph shows how the solubility of various chemicals varies with temperature. The solubility is measured as grams of dissolved chemical (solute) per 100 grams of water.
Under the assumption that the curves shown continue in the same general shape beyond the plotted area, use the drop-down menus to complete each of the following statements in the manner that most accurately reflects the information provided.
| Text Component | Literal Content | Simple Interpretation |
|---|---|---|
| Data Type | The graph shows how the solubility of various chemicals varies with temperature | The dataset is about how temperature changes affect how chemicals dissolve in water |
| Measurement Unit | The solubility is measured as grams of dissolved chemical (solute) per 100 grams of water | The y-axis shows grams of chemical that dissolve in 100g water |
| Variable Relationship | Solubility varies with temperature | Temperature (x-axis) changes the amount that dissolves (y-axis) |
| Chart Component | What's Shown | What This Tells Us |
|---|---|---|
| Chart Type | Multiple curves (lines), one per chemical | Enables comparison across chemicals at various temperatures |
| X-axis Range | Temperature from \(0°\mathrm{C}\) to \(100°\mathrm{C}\) in increments | Shows solubility behavior across a significant temperature span |
| Y-axis Range | Solubility from 0g to 150g per \(100\mathrm{g H_2O}\) | Different chemicals dissolve in much greater or lesser amounts |
| Curve Patterns | Most curves rise, some curve sharply upward; a few decrease (e.g., \(\mathrm{SO_2}\)) | Temperature generally increases solubility, but not for all chemicals |
| \(60°\mathrm{C}\) Chemical Solubility | KI is shown as most soluble at \(60°\mathrm{C}\) | KI is the most soluble of the listed chemicals at \(60°\mathrm{C}\) |
| \(\mathrm{NH_4Cl}\) and \(\mathrm{KClO_3}\) Trajectory | \(\mathrm{NH_4Cl}\) increases steeply, \(\mathrm{KClO_3}\) increases fastest at higher temperatures | Their curves may cross above \(100°\mathrm{C}\), so equal solubility is plausible there |
KI is the most soluble major chemical at \(60°\mathrm{C}\) among those listed. \(\mathrm{NH_4Cl}\) sharply increases in solubility with temperature and could reach the same solubility as \(\mathrm{KClO_3}\) at a temperature above \(100°\mathrm{C}\), assuming curve trends continue. Different chemical solubility curves show unique shapes, indicating variable responses to heating: most increase, but some (like \(\mathrm{SO_2}\)) decrease with temperature.
At \(60°\mathrm{C}\), the most soluble of the chemicals is most likely [BLANK].
There is most likely a temperature above \(100°\mathrm{C}\) at which \(\mathrm{NH_4Cl}\) is exactly as soluble as [BLANK].
Question 1 is answered by comparing actual solubility values at \(60°\mathrm{C}\), where KI stands out as the highest among the provided choices. Question 2 involves projecting existing curve trends, recognizing that only \(\mathrm{KClO_3}\)'s steeply increasing solubility makes it plausible to intersect \(\mathrm{NH_4Cl}\) above \(100°\mathrm{C}\).
The two questions are independent: one is a single-point comparison, and the other is a forward-looking trend analysis. You do not need the answer to one to work out the other.